Ethical issues in vaccine trial participation by adolescents: qualitative insights on family decision making from a human papillomavirus vaccine trial in Tanzania
Lucy Frost, Ms Tusajigwe Erio, Hilary Whitworth, Ms Graca Marwerwe, Richard Hayes, Kathy Baisley, Silvia de SanJosé, Deborah Watson-Jones, Kirstin Mitchell
BMC Medical Ethics, 20 November 2024
Open access
Abstract
Background
Research in children is essential for them to benefit from the outcomes of research but involvement must be weighed against potential harms. In many countries and circumstances, medical research legally requires parental consent until the age of 18 years, with poorly defined recommendations for assent prior to this. However, there is little research exploring how these decisions are made by families and the ethical implications of this.
Aim
To explore key ethical debates in decision-making for participation of children and adolescents in a human papillomavirus (HPV) vaccine trial.
Methods
Semi-structured interviews were undertaken with Tanzanian girls (aged 9–16 years) who had participated in an HPV vaccine trial (n = 13), their parents or guardians (n = 12), and girls together with their parents (in paired parent-child interviews) (n = 6). The interviews were analysed using thematic analysis. Interview data came from a qualitative acceptability study undertaken as part of the Dose Reduction Immunobridging and Safety Study of Two Human Papillomavirus (HPV) Vaccines in Tanzanian Girls (DoRIS) trial.
Results
Girls and parents desired collaborative decision-making, with parents ultimately making the decision to consent. However, girls wanted a larger part in decision-making. Decisions to consent involved many people, including extended social networks, the trial team, media outlets and healthcare professionals and this resulted in conflicts to be negotiated. Deciding where to place trust was central in participants and parents considering decisions to consent and overcoming rumours about trial involvement.
Conclusions
Existing models of decision-making help to understand dynamics between parents, adolescents and researchers but neglect the important wider social impacts and the fundamental nature of trust. Children’s roles in discussions can be evaluated using the principles of consent: autonomy, freedom and information. Concepts such as relational autonomy help to explain mechanisms families use to negotiate complex consent decisions. Whilst interviewees supported the maintenance of legal parental consent, researchers must design consent processes centring the child to ensure that whole family decision-making processes are supported.
Month: November 2024
Tools for effective patient education to manage outcome expectations in paediatric facial reanimation: a systematic review
Tools for effective patient education to manage outcome expectations in paediatric facial reanimation: a systematic review
Systematic Review
Dimitris Reissis, Cédric Zubler, Edel de Buitleir, Sam Brown, Jonathan Leckenby, Adriaan Grobbelaar
Plastic and Aesthetic Research, 30 October 2024
Open Access
Abstract
Aim
Informed consent for paediatric facial reanimation requires effective patient/parent education and involvement in a shared decision-making (SDM) process to help set their expectations and understanding from the outset. No article in the current literature has systematically reviewed the available tools for facilitating effective patient/parent education and the validity of informed consent in the context of paediatric facial reanimation.
Methods
A systematic literature review was undertaken, following the Preferred Reporting Items of Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines. MEDLINE via PubMed, Embase and Cochrane Library were searched and the results screened and reviewed in accordance with pre-defined inclusion and exclusion criteria.
Results
The initial search yielded 478 articles, of which only 4 fulfilled the study’s inclusion criteria. One cohort study evaluated qualitative feedback from patients and their relatives participating in a family education and support day for paediatric facial palsy, while another article from the same group reviewed the readability of online education resources. The remaining two articles represented educational reviews focusing on treatment and patient education based on expert opinion without providing original outcome data.
Conclusion
There is a paucity of evidence regarding patient/parent education to support the informed consent process for children undergoing paediatric facial reanimation. There remains a need for further resources and platforms to be developed that may support children and their parents in engaging in a SDM process, setting appropriate expectations, and providing valid informed consent for their surgery.
Prescribing contraceptives to minors without parental knowledge and consent
Prescribing contraceptives to minors without parental knowledge and consent
M Peled-Raz, O Goldstick
European Journal of Public Health, 28 October 2024
Abstract
Background
Sexually active adolescents may seek oral contraceptives without parental consent, posing challenges due to minors’ confidentiality and consent regulations. This is especially the case under the un-nuanced Israeli legal scheme regarding adolescents’ care.
Methods
Israeli OBGYNs were contacted through mailing lists and social media groups and asked to fill an online questionnaire regarding their experience and protocols concerning prescription of contraceptives to minors. They were also asked about their comprehension of the relevant legal obligations, the importance they ascribe to different ethical interests and considerations, as well as their training.
Results
Of the 177 responding gynecologists, 75% consulted minors about contraceptives during the past year, most of them without any training on providing care to adolescents. More than a third of respondents believed that parental involvement wasn’t legally required, while only 8% thought it mandatory for all minors under the age of 18. Most (75%) would ‘almost always’ prescribe contraceptives without parental knowledge upon request, while 20% never would. No correlation was found between respondents’ practices and their perception of the legal obligations. Participants agreed that the risk to the health of the minor due to having sex without contraceptives is of utmost importance. Those willing to prescribe gave greater weight to minor’s autonomy consideration, while those who do not prescribe were more concerned with the acts legal ramifications. The majority set the age of 15 as the threshold for consistently prescribing contraceptives to minors without parental involvement.
Conclusions
Access to contraceptives for mature minors without parental involvement is vital. There is great need for education and training for healthcare providers on providing medical treatment to adolescents, as well as for the development of policies and guidelines, addressing adolescents’ health disparities.
The ethical inadequacy of uninformed surrogate consent: advancing respect for persons in clinical research
The ethical inadequacy of uninformed surrogate consent: advancing respect for persons in clinical research
Robert R. Harrison
Theoretical Medicine and Bioethics, 10 November 2024
Abstract
In clinical research, decision-making capacity is often equated with unspecified conceptions of autonomy, and autonomy is often equated with personhood. On this view, the loss of decision-making capacity is seen as a loss of autonomy, and the loss of autonomy subsumes a loss of personhood. An ethical concern arises at the intersection of those philosophical considerations with the legal considerations in informed consent. Because persons with inadequate decision-making capacity cannot provide legally effective consent, enrollment in research can occur only if a surrogate gives permission on the person’s behalf. Federal regulations and resulting institutional policies allow permission from surrogates empowered under state law to consent to medical treatment procedures, typically in a hierarchy of legislatively prioritized relationships lacking regard for what the surrogate actually knows about the current research-related values and preferences of the potential subject. As a result, the research enterprise often countenances reliance on surrogates who have no relational or informational basis for an enrollment decision that aligns with the values and preferences of the subject. Arguing from the perspective that losing decision-making capacity does not alter the moral status of persons, and that respect for persons rather than respect for autonomy is the central ethical obligation, I assess the ethical implications of allowing persons with no knowledge of the values and preferences of the potential subject to make enrollment decisions, concluding that reliance on uninformed surrogates is not an ethically defensible approach to enrolling subjects in clinical research.
Electroconvulsive therapy and informed consent: navigating clinical efficacy and patient rights
Electroconvulsive therapy and informed consent: navigating clinical efficacy and patient rights
Conference Presentation
Anghel Claudia
Beyond borders: united for mental health 2024; Chişinău, Moldova, 10-13 October 2024
Abstract
Electroconvulsive Therapy (ECT) remains a highly effective treatment for severe mental health disorders, such as treatment-resistant schizophrenia and major depressive disorder. However, its use raises important ethical and legal concerns, particularly regarding informed consent. Balancing the clinical efficacy of ECT with patients’ rights to make informed decisions about their treatment is a complex challenge for mental health professionals. Informed consent is crucial, requiring that patients fully understand the potential benefits, risks, and side effects of ECT before agreeing to the procedure. Mental health care providers must ensure that patients are not only informed about the short- and long-term effects of ECT but also supported in their decisionmaking process. This includes addressing any cognitive impairments or mental health symptoms that may impact their capacity to give informed consent. Additionally, legal guardians or family members may be involved in the consent process, especially when patients are unable to provide it themselves. Ultimately, navigating the delicate balance between ensuring the clinical success of ECT and respecting patient autonomy is essential for ethical practice. Continued research into improving patient education and consent processes can help to understand the efficacy of ECT and the rights of them receiving this treatment.
Anosognosia in Alzheimer’s Disease: Clinical Psychology and Medico-Legal Issues. Informed Consent in Healthcare
Anosognosia in Alzheimer’s Disease: Clinical Psychology and Medico-Legal Issues. Informed Consent in Healthcare
Tomasello Letters, Miriana Ranno, Claudia Pitrone
New Medical Innovations and Research, 28 March 2024
Abstract
Insight or deficit awareness have been used interchangeably to refer the lack of knowledge or recognition of one’s deficit. Our aim was to investigate whether this lack could influence Alzheimer’s disease patients’ ability to understand and do.
Disease awareness is a phenomenon that in recent years is obtaining an increasing interest in a clinical and research point of view. It has important implications on patient care and management. The present study is aimed to contribute to the comprehension of disturbing awareness in patients with Alzheimer disease, and provided a starting point on a complex disease linked to medical and psychological scopes but also involve Bioethics and Law.
Informed consent and decision-making skills
Informed consent is a fundamental prerequisite of every medical act and the autonomy of a patient, in the fullness of his ability to decide on treatments and possible therapeutic treatments. Presupposition of informed consent, beyond the information (well given and well understood) and freedom (absence of conditioning factors or at least awareness of their presence), is the ability to decide. The ability to decide on medical treatment is inherent in the legal concept of capacity to act (Art. 2 of the CC). The definition, proposed by Wong et al. (1999) (10), provides an indication of the relationships between the ability of the individual and the society around him: “capacity is what distinguishes a person, who is able to make a decision and whose choice must be respected, regardless of the reasonableness of the choice, by a person for whom decisions must be made by others”. There are cases where a person may no longer be able to manage his or her current account but may be able to give his or her consent to simple medical treatment. The ability to decide must be presumed, until proven otherwise. Dementia is, therefore, a risk factor for incapacity, but it does not inevitably involve it. The ability (or inability) is always relative to a certain task. For example, a person may be able to make a decision for simple medical treatment but not be able to discern complex alternatives with different risk/benefit profiles…
Evaluating the Informed Consent Process: Insights from Post-Operative Experiences in Pharmaceutical Care
Evaluating the Informed Consent Process: Insights from Post-Operative Experiences in Pharmaceutical Care
Research Article
Muhammad Ajmal, Arslan Wajid, Zahra Rafique, Ahsan Sikandar Khan, Abdul Rehman Saddiq, Muhammad Sulaiman, Muhammad Aqeel Sultan, Usman Wajid
History of Medicine, 30 September 2024
Abstract
Background
An informed consent must be obtained legally and ethically before invasive or high-risk therapeutic procedures are performed. It is defined as the “process of communication between a patients and healthcare professionals that leads in the patient’s permission or agreement to undergo any specific medical procedure.
Aim
To investigate informed consent’s practices and determine whether the persons who have signed for surgical treatments have a sufficient understanding about the process of informed consent.
Methodology
It was a descriptive cross-sectional study that was conducted at the Rehman Medical Institute (RMI) Peshawar. Using Simple Random Probability Sampling Technique; a sample of 108 surgical patients was recruited. Data was collected using closed ended interview schedule. The validity of the redesigned instrument was evaluated by a panel of specialists, including a research supervisor and surgical practitioners. To analyze data a descriptive statistic will be used. The computer’s software, Statistical Package for Social Science (SPSS version 20) will be used for data analysis and interpretation.
Result
The sample size was 108 patients, with a 100% response rate. A total of 108 patients (89 male and 19 female) were randomly selected for post-operative interviews. Out of 108 patients, all the patients gave and signed the pre-operative informed consent process form on their own. Only 21 (19.44%) patients already knew about the informed consent process because they had almost a bachelor’s degree education. Only 31 patients (28.70%) read and fully understood the surgical informed consent process form. And 106 (98.14%) had their consent taken by a young doctor rather than the surgeon who would be doing the surgery.
Conclusion
Our study revealed that quality of informed consent process is limited at RMI Hayatabad Peshawar, due to surgeons making little or no attempt to educate their patients on this subject and the informed consent form is only available in English, with no verified translation into the patient’s mother tongue.
Assessing the Process of Written Informed Consent for Surgical Procedures among Inpatients: A Cross-sectional Study from a Tertiary Care Teaching Hospital in Southern India
Assessing the Process of Written Informed Consent for Surgical Procedures among Inpatients: A Cross-sectional Study from a Tertiary Care Teaching Hospital in Southern India
V. Dinesh, Imaad Mohammed Ismail, Kahkashan Azeez
Journal of Clinical & Diagnostic Research, 2024
Abstract
Introduction
Informed Consent (IC) is a decision-making process wherein patients are provided with all necessary information regarding treatment to make an uncoerced, educated choice. There are gaps in the implementation of the IC process that need to be identified and addressed.
Aim
To estimate the proportion of patients/surrogates who read, understood, and signed the IC form before undergoing surgical procedures; to identify the different healthcare team members involved in explaining the IC form; to evaluate the extent to which different components of the IC form were explained to patients/surrogates; and to determine the influence of the IC form on surgical decision-making, and the overall satisfaction with the IC process.
Materials and Methods
This cross-sectional study was conducted at a tertiary care hospital in the Dakshina Kannada District of Southern India from April 2020 to March 2021. It included 100 adult patients admitted to the postsurgical wards of general surgery, orthopaedics, obstetrics and gynaecology, otorhinolaryngology, and ophthalmology. Ethical clearance was obtained from the Institutional Ethics Committee. The parameters studied included socio-demographic variables, administration of the IC form, details on the person explaining the IC form along with its content, and the influence of the IC form on decision making, as well as overall satisfaction with the IC process. Data were collected using a predesigned questionnaire and analysed using descriptive statistics in Statistical Package for the Social Sciences (SPSS) version 27.0. Categorical variables were presented as frequencies and proportions, whereas continuous variables were presented as means and standard deviations.
Results
All participants received the IC form; however, only 21% read, understood, and signed it. The explanation of the IC form was given to 59% of the patients, with only 15% of these explanations provided by the treating surgeon. The components of the IC form, such as the surgical procedure and its benefits, were explained to the majority of the patients; however, the risks of the surgical procedure and alternative options were explained to only 53% and 7% of patients, respectively. The IC form had a minor influence on surgical decision-making for 61% of patients, and 43% expressed satisfaction with the IC process.
Conclusion
The study revealed that the implementation of IC was inadequate. Surgeons should provide and explain the IC form well in advance, allowing time for patients to read, understand, and clarify their doubts. Hospital Ethics Committees need to enforce strict adherence to IC guidelines to ensure informed decision-making.
Consent for organ donation: a case study in the light of bioethics
Consent for organ donation: a case study in the light of bioethics
Health Sciences
Kelly C.B. Gomes, Mary R.G. Esperandio, José E. De Siqueira, José R. Goldim
The Annals of the Brazilian Academy of Sciences, 2024
Abstract
Fewer donations are being made in Brazil to meet the growing organ demand. Organ donation in Brazil reached an average of 53% consent. However, hospitals in Paraná have reached a level of 94.2%. What reasons could be given for these levels? Accordingly, this study aimed to understand the causes involved in decision-making to donate organs. The methodology used was qualitative based on a case study. Data was collected at a hospital in Toledo, a city in Paraná, through documentary research and semi-structured interviews with two distinct groups: professionals responsible for the family approach to donation and five families consenting to donation. The search for data was restricted to the period between 2015 and 2023. Data analysis used Bardin’s content analysis. The results were organized into four categories in the first group, and two categories in the second group, suggesting that aspects linked to bioethical references present in the interview, such as beneficence and autonomy, contribute to the emergence of high rates of family consent for organ donation in the hospital studied. It is recommended for future research to test successful interview models to reverse the current organ donation rates in Brazil.
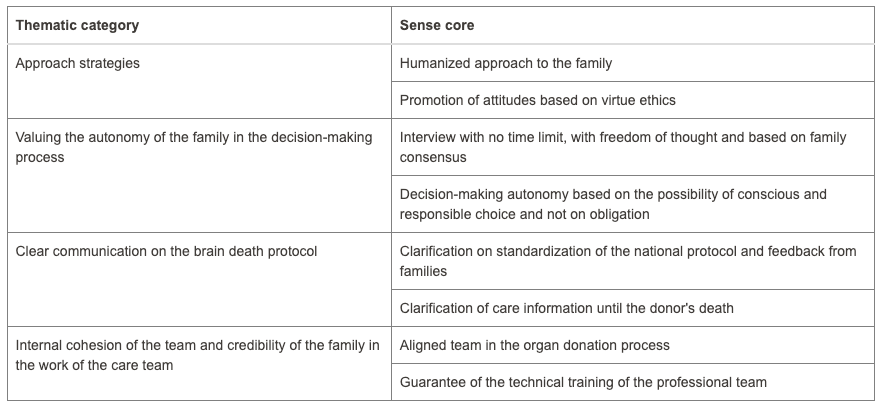
Four categories from the first group as mentioned in the abstract
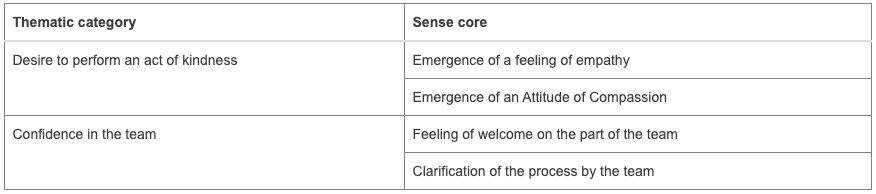
Two categories from the second group as mentioned in the abstract
Clinicians’ experiences of obtaining informed consent for research and treatment: a nested qualitative study from Pakistan
Clinicians’ experiences of obtaining informed consent for research and treatment: a nested qualitative study from Pakistan
Research
Rakhshi Memon, Muqaddas Asif, Bushra Ali Shah, Tayyeba Kiran, Ameer B Khoso, Sehrish Tofique, Jahanara Miah, Ayesha Ahmad, Imran Chaudhry, Nasim Chaudhry, Nusrat Husain, Sarah J L Edwards
BMC Medical Ethics, 15 November 2024
Open access
Abstract
Background
Informed consent is considered to be the standard method for respecting the autonomy of individual participants in research and practices and is thought to be based on several conditions: (1) providing information on the purpose of the research or a specific treatment, what it will entail, (2) the participants being mentally competent to understand the information and weigh it in the balance, and (3) the participants to be free from coercion. While there are studies of informed consent in other countries, especially Low and Middle Income Countries (LMICs), this study explored the experiences of clinicians regarding the process of obtaining informed consent to participate in a Randomised Controlled Trial (RCT) in particular and treatment in general in healthcare settings, both general and mental health, specifically focusing on the tension between individualistic concept of autonomy and collectivist values in cultures such as Pakistan.
Methods
Qualitative interviews with 20 clinicians from healthcare settings in Pakistan who also served as recruiters in a suicide prevention RCT in Pakistan. The interviews were guided by semi-structured topic guide. All interviews were audio-recorded and transcribed verbatim.
Results
The interviews revealed that shared decision making was more morally important than individual autonomy, the role of the family played a dominant part in the consent-taking procedure, the decision of the elder and/or family patriarch took prominence, and that clinician-researchers encountered significant challenges in consent process in Pakistan, while recruiting patients into the trial as well as during routine treatment processes in healthcare settings. Four distinct themes emerged which were (1) Family deciding for patients, (2) Benefits of involving family in consent process, (3) Gender disparity in consent process, (4) Challenges experienced by clinician-researchers during consent process in Pakistan.
Conclusions
The concept of consent is generally considered important in many cultures, however, there are two strands of understanding. There seems to be consensus that participant agreement is necessary to protect the participant but with regards to autonomy there are significant cultural differences whether it is the right for autonomy of the individual (individualistic concept) or family, community, or expert authority in other cultures. In Pakistan clinician-researchers sometimes preferred one approach and sometimes the other as they appreciated the interests of the patient to be.