Center for Informed Consent Integrity – Webinar Series
Webinar of 20 October 2021
Webinar Topics
:: Emerging consent/choice/refusal issues for young persons in the COVID vaccines context globally
Host/Speakers/Presenters
David Curry, MS
President & CEO, GE2P2 Global Foundation
Call Summary
On October 20th 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent. Foundation President David Curry, who has led the Foundation’s Center for Vaccine Ethics and Policy since 2008, opened the session with a discussion on the intersection of limited supply of WHO/SRA [Stringent Regulatory Authority] reviewed vaccines, licensing and use of “questionable” vaccines outside WHO/SRA review, growing vaccine hesitancy/refusal as a global challenges, growing use of mandates of various types but specifically involving young persons and a return to FTF education, and limited options for young people who find themselves with few options to exercise. After the initial presentation participants on the call shared local perspectives.
Recording
Resources
WHO-2019-nCoV-Policy-brief-Mandatory-vaccination-2021.1-eng
Webinar of 15 September 2021
Webinar Topics
:: Mainstreaming informed consent for genomic sequencing: A call for action
Host/Speakers/Presenters
Webinar Host – David Curry, MS
President & CEO, GE2P2 Global Foundation
Eline Bunnik, PhD
Erasmus University
Call Summary
On September 15th 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent. Dr. Eline M. Bunnik of Erasmus University shared perspectives from her May 2021 paper Mainstreaming informed consent for genomic sequencing: A call for action. During her presentation Dr. Bunnik also provided detail on the consortium and the help desk capability that was formed during this research. Her presentation was followed by an open discussion involving all call participants.
Recording
Resources
Slides: GE2P2 Global Fdn_CICI Webinar Series_15 Sep 2021
Help Desk: https://elsi.health-ri.nl/
Webinar of 21 July 2021
Webinar Topics
:: Informed Consent in the Council for International Organizations of Medical Sciences (CIOMS) guidance on Clinical research in resource-limited settings
Host/Speakers/Presenters
Webinar Host – David Curry, MS
President & CEO, GE2P2 Global Foundation
Paige Fitzsimmons, MA
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
Dónal O’Mathúna, PhD
Senior Fellow and Director, GE2P2 Global Foundation
Associate Professor, College of Nursing and Center for Bioethics, The Ohio State University
Getnet Yimer, MD, PhD
Director, Global One Health Eastern Africa Office
Call Summary
On July 21st 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent. This call focused on the newly released CIOMS Guidance on clinical research in resource-limited settings.
David Curry opened the call with a high-level overview of the guidance and its relationship to the 2016 CIOMS guidance: International Ethical Guidelines for Health-related Research Involving Humans.
Paige Fitzsimmons followed with a brief overview of how informed consent is treated in the guidance. Getnet Yimer provided observations and reflections on this guidance and associated challenges with implementation from a field research and ethics review board perspective. Dónal O’Mathúna closed the discussion by examining how community engagement is treated in the new guidance.
This panel was followed by open discussion amongst all call participants.
Recording
Resources
CIOMS Guidance on Clinical Research in Resource-Limited Settings_June 2021
Community engagement and building trust to resolve ethical challenges during humanitarian crises- experience from the CAGED study
Interventions to prevent misconduct and promote integrity in research and publication (Review)
Webinar of 16 June 2021
Webinar Topics
:: Closing the loop on consent: from initial decision, continued participation, through to sharing of results
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
David Curry, MS
President & CEO, GE2P2 Global Foundation
Katie Gillies, PhD
Director, Healthcare Assessment Program, Health Services Research Unit, University of Aberdeen
Call Summary
On June 16th 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent.
Dr. Katie Gillies of the University of Aberdeen spoke about her work to strengthen the informed consent by developing an understanding of what information matters most to trial participants. She then spoke about how this understanding can lead to increased participant retention and further work to be done.
Dr. Gillies’ presentation was followed by a rich discussion amongst all call participants.
Recording
References
What potential research participants want to know about research: a systematic review
Helen Michelle Kirkby, Melanie Calvert, Heather Draper, Thomas Keeley, Sue Wilson
BMJ Open, 30 May 2012
Relative importance of informational items in participant information leaflets for trials: a Q-methodology approach
Karen Innes, Seonaidh Cotton, Marion K Campbell, Jim Elliott, Katie Gillies
BMJ Open, 5 September 2018
Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation
Katie Gillies, Wan Huang, Zoë Skea, Jamie Brehaut, Seonaidh Cotton
Trials, 18 February 2014
Decision aids for people considering taking part in clinical trials (Review)
Gillies K, Cotton SC, Brehaut JC, Politi MC, Skea Z
Cochrane Database of Systematic Reviews, 2015
Determining information for inclusion in a decision-support intervention for clinical trial participation: A modified Delphi approach.
Katie Gillies, Zoë C Skea, Sara J MacLennan, Craig R Ramsay, Marion K Campbell
Clinical Trials, 4 November 2013
Decision aids for randomised controlled trials: a qualitative exploration of stakeholders’ views
Katie Gillies, Zoë C Skea, Marion K Campbell
BMJ Open, 19 August 2014
Development and evaluation of decision aids for people considering taking part in a clinical trial: a conceptual framework
Katie Gillies, Marion K. Campbell
Trials, 5 July 2019
An international core outcome set for evaluating interventions to improve informed consent to clinical trials: The ELICIT Study
Katie Gillies, Paula R Williamson, Vikki A Entwistle, Heidi Gardner, Shaun Treweek, Marion K Campbell
Journal of Clinical Epidemiology, 26 February 2021
Exploring non-retention in clinical trials: a meta-ethnographic synthesis of studies reporting participant reasons for drop out
Zoë C Skea, Rumana Newlands, Katie Gillies
BMJ Open, 3 June 2019
An embedded mixed-methods study highlighted a lack of discussions on retention in clinical trial consultations
Pamela Tunji-Ajayi, Eilidh M. Duncan, Katie Gillies
Journal of Clinical Epidemiology, 1 July 2020
Consent revisited: the impact of return of results on participants’ views and expectations about trial participation
Carolyn Tarrant, Clare Jackson, Mary Dixon-Woods, Sarah McNicol, Sara Kenyon, Natalie Armstrong
Health Expectations, 30 April 2015
::::::
::::::
Webinar of 21 April 2021
Webinar Topics
:: Posting of Informed Consent Content on Clinical Trials Registries
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
David Curry
President & CEO, GE2P2 Global Foundation
Barbara Redman, PhD
Senior, Fellow; Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
Jan Jaeger, PhD
Fellow, GE2P2 Global Foundation
Call Summary
On April 21st 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent.
Foundation president David Curry opened the call with a short presentation followed by a panel discussion with Barbara Redman and Jan Jaeger. The discussion was focused on issues and opportunities around the posting of informed consent content [ICFs+] on clinical trial registries as a means to enhance transparency and strengthen consent in trials overall.
In particular, the GE2P2 team discussed its continuing review of the revised Common Rule regulation at 45 CFR 46.116(h) requiring posting of ICF content on either clincialtrials.gov or regulations.gov for trials which receive U.S. federal support [Common Rule-agencies]. Over 3,000 ICF documents have already been posted in clinical trial records covering a broad range of disease areas/indications. GE2P2 is refining its view that this posting requirement represents a normative standard that should be engaged beyond U.S. federally-supported trials, especially in the gene editing/gene therapy development.
The latter half of the call consisted of a rich discussion amongst all call participants.
Recording
::::::
::::::
Webinar of 17 March 2021
Webinar Topics
:: Informed consent for neonatal trials – Practical points to consider
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
[Vancouver, Canada]
David Curry
President & CEO, GE2P2 Global Foundation
[Philadelphia, USA]
Dr. Beate Aurich
Institut National de la Santé et de la Recherche Médicale (INSERM)
[Paris, France]
Dr. Eric Vermeulen
Dutch Patient Association for Rare and Genetic Diseases (VSOP)
[Soest, Netherlands]
Call Summary
On March 17th 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity continued a series of webinars focused on integrity in informed consent.
Invited speakers Dr. Beate Aurich of the Institut National de la Santé et de la Recherche Médicale (INSERM) in Paris, France, and Dr. Eric Vermeulen of the Dutch Patient Association for Rare and Genetic Diseases (VSOP) in Soest, The Netherlands, presented Informed consent for neonatal trials – Practical points to consider.
The presentation was followed by a rich discussion with call participants regarding areas such as parental consent, assent and reconsent, and the role of patient and parent involvement in trials and the design of the informed consent process.
Recording
Resources
Informed consent for neonatal trials- practical points to consider and a check list
::::::
::::::
Webinar of 20 January 2021
Webinar Topics
:: PPMD Gene Therapy Preference Study: Eliciting patient and caregiver preference for emerging gene therapies
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
David Curry
President & CEO, GE2P2 Global Foundation
Pat Furlong
President & CEO, Parent Project Muscular Dystrophy
Ryan Fischer
Chief Advocacy Officer, Parent Project Muscular Dystrophy
Call Summary
On January 20th 2021 the GE2P2 Global Foundation’s Center for Informed Consent Integrity held the fourth in a series of webinars regarding integrity in informed consent.
Invited speakers Pat Furlong, President & CEO, and Ryan Fischer, Chief Advocacy Officer, of Parent Project Muscular Dystrophy (PPMD) presented PPMD Gene Therapy Preference Study: Eliciting patient and caregiver preference for emerging gene therapies.
The presentation focused on their work understanding informed consent in rare disease, specifically Duchenne Muscular Dystrophy, and the quantification of patient and caregiver preferences in gene therapy studies to help inform research and researchers. The presentation was followed by a question period and discussion with call participants
Recording
Resources
Presentation Slide Deck: PPMD – Gene Therapy Preference Study Results
::::::
::::::
Webinar of 16 December 2020
Webinar Topics:
:: Inconvenient Imperatives – COVID-19 Immunization under Emergency Use:: Consent/Refusal, Mandates, Certificates
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons, MS
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
[London, UK]
David Curry, MS
President & CEO, GE2P2 Global Foundation
[Philadelphia, USA]
Call Summary
On December 16th 2020 the GE2P2 Global Foundation’s Center for Informed Consent Integrity held the third in a series of webinars regarding integrity in informed consent.
Foundation President & CEO David Curry opened the call with a summary of the initial, highly-limited COVID-19 vaccine “availability” via emergency use [EUA/EUL+] and other pre-licensure/pre-“full approval” mechanisms we are now seeing in some countries [U.K., Canada, USA+].
Among other topics, David introduced a new quadrant analysis for informed consent for vaccines [below], and provided an overview of the current information sheet that is being provided to healthcare workers and long-term care facility residents in the US who may be considering receiving the Pfizer vaccine at this time.
This initial overview was followed by a rich discussion amongst call participants regarding for informed consent for COVID-19 vaccines followed this initial overview.
Call Recording
Resources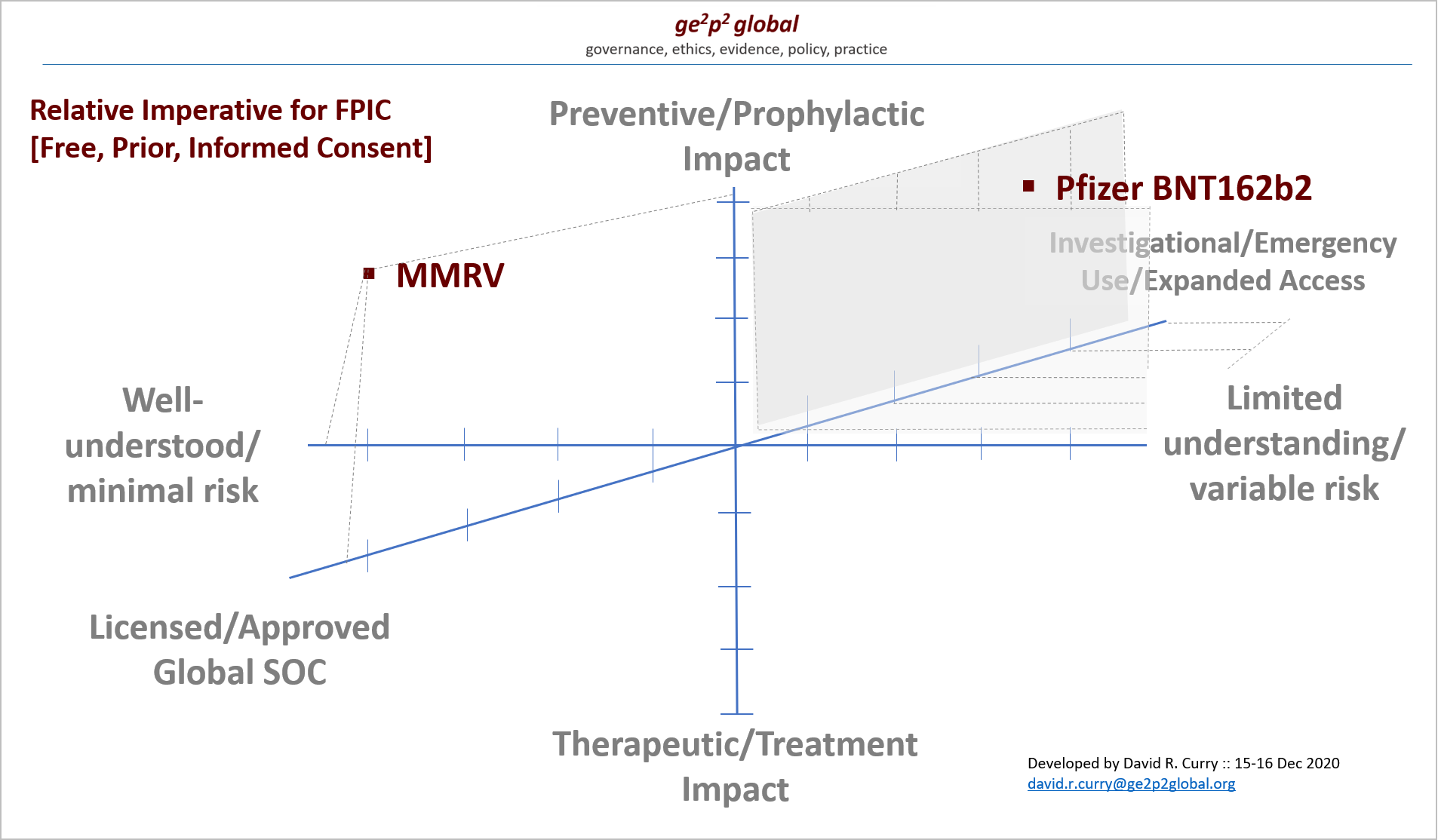
GE2P2 Global Public Comment — CDC-ACIP Meeting on Pfizer Covid Vaccine Deployment under Emergency Use – 12 December 2020
GE2P2 Global – CVEP_ACIP Public Comment on Pfizer COVID Vaccine Recommendation_12 Dec 2020
FDA
:: Pfizer-BioNTech COVID-19 Vaccine EUA Fact Sheet for Recipients
UK
:: Information_for_UK_recipients_on_Pfizer_BioNTech_COVID-19_vaccine 12-7-20
:: UK_Emergency Use Reg_2020
WHO
:: WHO – Emergency Use Listing Procedure
Version 9 January 2020
WHO_EUA Listing procedure_Overview_Jan 2020
:: WHO – CONSIDERATIONS FOR EVALUATION OF COVID19 VACCINES
Points to consider for manufacturers of COVID19 vaccines
Version 24 September 2020
WHO-EUAL_JAN 2020_eulprocedure
WHO SAGE Values Framework MASTER 2020 09 07_clean-1
Literature
Lancet_EUA Vax_Upshur__NoP2020 IIS0140673620323370 WHO_Evaluation_Covid_Vaccine_PQ-EUL_Sep 2020 01
Grady et al_Vaccine_COVID Ethics_Sep 2020_1-s2.0-S0264410X20310483-main
::::::
::::::
Webinar of 18 November 2020
Webinar Topics:
:: Informed Consent – Article 5 of the UN Convention on the Rights of the Child
:: GE2P2 Global Foundation and Center Update
Host/Speakers/Presenters
Webinar Host – Paige Fitzsimmons, MS
Associate Director, Center for Informed Consent Integrity, GE2P2 Global Foundation
[London. UK]
David Curry, MS
President & CEO, GE2P2 Global Foundation
[Philadelphia, USA]
Aoife Daly, PhD
Lecturer in Law, University College Cork
[Cork, Ireland]
Sheila Varadan, PhD Candidate
Fellow, GE2P2 Global Foundation
[Bangkok, Thailand]
Call Summary
On November 19th 2020 the GE2P2 Global Foundation’s Center for Informed Consent Integrity held the second in a series of webinars regarding integrity in informed consent.
Foundation President & CEO David Curry opened the call with a short update on current work of the Center for Informed Consent integrity, including the ongoing discussion regarding informed consent in COVID-19 vaccine/therapy provision and the informed consent for genomic medicine initiative.
Following this, invited speaker Aoife Daly, PhD, Lecturer in Law, University College Cork, spoke about assessing and understanding children’s capacity to provide informed consent. She spoke to her rights based approach to determining children’s capacity using various concepts from the UN Convention on the Rights of the Child.
Sheila Varadan, PhD Candidate, Leiden University, Fellow, GE2P2 Global Foundation, discussed the role of the UN Convention on the Rights of the Child in medical research settings, suggesting that article 5 of the UN Convention could offer support researchers navigating parent-child decision making in the proxy informed consent process.
Call Recording
Rescources
Assessing Children’s Capacity: Reconceptualising our Understanding through the UN Convention on the Rights of the Child
Research Article
Aoife Daly
The International Journal of Children’s Rights, 24 August 2020; 28(3) pp 471-499
Open Access
Abstract
This article seeks to reconceptualise approaches to assessing children’s capacity, particularly in light of Article 5 of the crc, which enshrines the principle of the evolving capacities of the child. Professionals regularly assess children’s capacity, for example when doctors treat children, or when lawyers represent child clients. They usually do this assessment intuitively however, as there is little guidance on how assessment should work in practice. Medical law in England and Wales serves as a case study to examine law and practice as well as challenges in the area. It is concluded that it may not necessarily be possible objectively to measure children’s capacity, and it may need to be done intuitively. Yet it should be done via a process which is rights-based. An approach to children’s capacity is proposed through four concepts based on the UN Convention on the Rights of the Child: Autonomy, Evidence, Support and Protection.
PDF: Assessing Children’s Capacity
Article 5: The Role of Parents in the Proxy Informed Consent Process in Medical Research involving Children
Research Article
Sheila Varadan
The International Journal of Children’s Rights 24 August 2020; 28(3) pp 521-546
Open Access
Abstract
Medical research involving child subjects has led to advances in medicine that have dramatically improved the lives, health and well-being of children. Yet, determining when and under what conditions a child should be enrolled in medical research remains an ethically vexing question in research ethics. At the crux of the issue is the free and informed consent of the child participant. A child, who is presumed legally incompetent, or lacks sufficient understanding to exercise autonomous decision-making, will not be able to express free and informed consent in the research setting. Rather than exclude all such children from medical research, a parent (or legal guardian) is designated as a proxy to consent on the child’s behalf. However, the concept of proxy informed consent and the framework for its implementation present practical and ethical challenges for researchers, particularly in navigating the relationship between proxy decision-makers and child subjects in the medical research setting. Article 5 of the uncrc may offer guidance on this point: (1) it places boundaries around how parental authority should be exercised; (2) it offers a model for parent-child decision-making that is participatory, collaborative and linked to the child’s enjoyment of rights under the uncrc; (3) it respects and supports the autonomy of child participants by recognising their evolving capacities to give informed consent. This paper concludes that greater consideration should be given to Article 5 as a complementary framework for researchers engaged in medical research involving children.
PDF: Article 5- The Role of Parents in the Proxy Informed Consent Process in Medical Research involving Children
Editorial [Introduction to the Special Issue]
By: Brian Sloan and Claire Fenton-Glynn Pages: 439–445
::::::
For separate discussion if time allows:
What Do you Mean by “Informed Consent”? Ethics in Economic Development Research
Featured Article
Anna Josephson, Melinda Smale
Applied Economic Perspectives and Policy, 27 October 2020
Abstract
The ethical conduct of research requires the informed consent and voluntary participation of research participants. Institutional Review Boards (IRBs) work to ensure that these ethical standards are met. However, incongruities in perspective and practice exist across regions. In this article, we focus on informed consent as practiced by agricultural and applied economists, with emphasis on research conducted in low income and/or developing countries. IRB regulations are clear but heterogeneous, emphasizing process rather than outcome. The lack of IRBs and institutional reviews in some contexts and the particulars of the principles employed in others may fail to adequately protect research participants.
PDF: IC in Econ Development Research_Nov 2020_AEPP_aepp.13112
::::::
::::::
Webinar of 19 May 2020
Webinar Topics:
:: GE2P2 Global Foundation and Center Overview
:: Presentation on Informed Consent in the Arab Region
Speakers/Presenters
David Curry, MS
President & CEO, GE2P2 Global Foundation
Thalia Arawi, PhD
Founding Director, Salim El-Hoss Bioethics & Professionalism Program (SHBPP), American University of Beirut
Senior Fellow, GE2P2 Global Foundation
Call Summary
On May 19th 2020 the GE2P2 Global Foundation’s Center for Informed Consent Integrity held the first in a series of webinars regarding informed consent integrity.
Foundation President & CEO David Curry opened the call with a short overview of the Foundation and the Center for Informed Consent Integrity itself. He spoke about current projects and strategic initiatives the Center has underway including work focused on informed consent in compassionate/expanded access for investigational medicines and therapies, and informed consent in the genomic medicine era
Following this, invited speaker Thalia Arawi, PhD, Founding Director of the Salim El-Hoss Bioethics & Professionalism Program (SHBPP) and Faculty of Medicine, American University of Beirut & Medical Center, and Senior Fellow of the GE2P2 Global Foundation, spoke about informed consent in the Arab Region. Her talk focused on two themes: clinical ethics and research ethics in general, and special challenges encountered when working in humanitarian contexts. In support of this presentation, David Curry noted three recent articles which have been captured in the resources section below. Dr. Arawi’s comments begin at 0:31:00 in the following recording.
Call Recording
This presentation contains slides which are proprietary to Dr. Thalia Arawi
IC Open Forum
The call agenda included time for an “open forum” inviting participants to share new ideas, issues, and new projects and research underway. While we had to defer on this agenda item on this first call due to time constraints, we invite you to share any thoughts by email to paige.fitzsimmons@ge2p2global.org and we can respond immediately or on future calls.
Resources
Journal Articles/Reports/Announcements Referenced
Knowledge and attitudes of physicians toward research ethics and scientific misconduct in Lebanon
Research Article
Bilal Azakir, Hassan Mobarak, Sami Al Najjar, Azza Abou El Naga & Najlaa Mashaal
BMC Medical Ethics, 14 May 2020; 21(39)
Open Access
Abstract
Background
Despite the implementation of codes and declarations of medical research ethics, unethical behavior is still reported among researchers. Most of the medical faculties have included topics related to medical research ethics and developed ethical committees; yet, in some cases, unethical behaviors are still observed, and many obstacles are still conferring to applying these guidelines.
Methods
This cross-sectional questionnaire-based study was conducted by interviewing randomly selected 331 Lebanese physicians across Lebanon, to assess their awareness, knowledge and attitudes on practice regarding international and national research ethics guidelines (Lebanese decrees/Laws and CNRS chart of ethics) and scientific misconduct and misbehaviors.
Results
Our results revealed that although majority of participants declared familiar with ethical principles governing research that involves human subjects (79.5%), the overall mean score achieved on their knowledge questions was 46%. Only 27.4% are aware of the presence of the Lebanese National Consultative Committee on Ethics (LNCCE), with only half of them aware of its functions and only 25.7% know about the charter of ethics and guiding principles of scientific research in Lebanon. Significant higher levels of research ethics knowledge were recorded among Ph.D. degree-holding subjects, higher university positions as in professors, research ethics trainings-attendees, and physicians with prior research experience. A significant correlation was observed between knowledge of research ethics principles and positive attitudes toward research ethics principles. Noteworthy, we found that more than one third of participants have reported witnessing scientific misconduct and misbehaviors at some period of their careers.
Conclusions
The presence of low mean awareness levels regarding research ethical principles among the study population of physicians and high levels of perception of scientific misconduct raises concern on the importance of implementing proper training for physicians on research ethics.
Conducting operational research in humanitarian settings: is there a shared path for humanitarians, national public health authorities and academics?
Debate
Enrica Leresche, Claudia Truppa, Christophe Martin, Ariana Marnicio, Rodolfo Rossi, Carla Zmeter, Hilda Harb, Randa Sami Hamadeh & Jennifer Leaning
Conflict and Health, 13 May 2020; 14(25)
Abstract
In humanitarian contexts, it is a difficult and multi-faceted task to enlist academics, humanitarian actors and health authorities in a collaborative research effort. The lack of research in such settings has been widely described in the past decade, but few have analysed the challenges in building strong and balanced research partnerships. The major issues include considering operational priorities, ethical imperatives and power differentials. This paper analyses in two steps a collaborative empirical endeavour to assess health service utilization by Syrian refugee and Lebanese women undertaken by the International Committee of the Red Cross (ICRC), the Lebanese Ministry of Public Health (MoPH) and the Harvard François-Xavier Bagnoud (FXB) Center.
First, based on challenges documented in the literature, we shed light on how we negotiated appropriate research questions, methodologies, bias analyses, resource availability, population specificities, security, logistics, funding, ethical issues and organizational cultures throughout the partnership.
Second, we describe how the negotiations required each partner to go outside their comfort zones. For the academics, the drivers to engage included the intellectual value of the collaboration, the readiness of the operational partners to conduct an empirical investigation and the possibility that such work might lead to a better understanding in public health terms of how the response met population needs. For actors responding to the humanitarian crisis (the ICRC and the MOPH), participating in a technical collaboration permitted methodological issues to be worked through in the context of deliberations within the wider epistemic community.
We find that when they collaborate, academics, humanitarian actors and health authorities deploy their respective complementarities to build a more comprehensive approach. Barriers such as the lack of uptake of research results or weak links to the existing literature were overcome by giving space to define research questions and develop a longer-term collaboration involving individual and institutional learning. There is the need ahead of time to create balanced decision-making mechanisms, allow for relative financial autonomy, and define organizational responsibilities. Ultimately, mutual respect, trust and the recognition of each other’s expertise formed the basis of an initiative that served to better understand populations affected by conflict and meet their needs.
Ethical framework for COVID-19 response in the Arab region: views and recommendations from the experts
UNESCO, 6 May 2020
Open Access
Excerpt
As the COVID-19 pandemic has put states, public health systems, economies, societies, communities, and individuals under utmost pressure, many questions have arisen about the norms and criteria that could guide sound decision-making process in response to the emergency.
In as much as the COVID-19 is a “global health” challenge, it indeed also raises fundamental and difficult questions pertaining to human rights, social justice, codes of ethics, and environmental ethics. The Arab States region is no exception to this challenge.
As stated by the UNESCO International Bioethics Committee (IBC) and the UNESCO World Commission on the Ethics of Scientific Knowledge and Technology (COMEST), “a bioethics and ethics of science and technology perspective, rooted in human rights, should play a key role in the context of this challenging pandemic”.[1] In this context, UNESCO considers it is vital to provide solid grounds for collective reflections on some of the following ethical and social dimensions, pertaining both to the medical treatment, and to the prevention and containment policies put in place by various states.
Just as a “whole-of-society” and “urgent” action is required, “multidisciplinary” analysis and recommendations are also key to understand and address the pandemic itself, plan the de-confinement, as well as to reflect on lessons learned for a post-COVID society over the medium and long terms…
[See bibliography of 15 articles at title link]