Qualitative Assessment of Proposed Visual Key Information Pages for Informed Consent
Krista E. Cooksey, Eliana Goldstein, Clara Lee, Jessica Mozersky, Kimberly A. Kaphingst, Victor Catalan Gallegos, Mary C. Politi
Journal of Clinical and Translational Science, 21 November 2024
Open Access
Abstract
Introduction
The 2018 Common Rule revision intended to improve informed consent by recommending a concise key information (KI) section, yet provided little guidance about how to describe KI. We developed innovative, visual KI templates with attention to health literacy and visual design principles. We explored end users’ attitudes, beliefs, and institutional policies that could affect implementing visual KI pages.
Materials and Methods
From October 2023-April 2024, we conducted semi-structured interviews with principal investigators, research staff, institutional review board (IRB) personnel, including those in oversight/management, and community partners. 40 participants from 3 academic institutions (in the Midwest, Southeast, and Mountain West) viewed example KI pages and completed interviews. We coded written transcripts inductively and deductively based on the capability, opportunity, and motivation to change behavior (COM-B) framework. Data were analyzed using content analysis and organized thematically.
Results
Participants responded positively to the visual KI examples. They discussed potential benefits including improving information processing and understanding of study procedures, diversity in research, trust in research, and study workflow. They also described potential challenges to consider before widespread implementation: IRBs’ interpretations of federal guidelines, possible impact on the IRB submission processes, the effort/skill required to develop visuals, and difficulty succinctly communicating study risks. There was no consensus about when to use visual KI during consent, and some wondered if they were feasible for all study types.
Discussion
Visual KI offers a promising solution to long-standing informed consent challenges. Future work can explore resources and training to address challenges and promote widespread use.
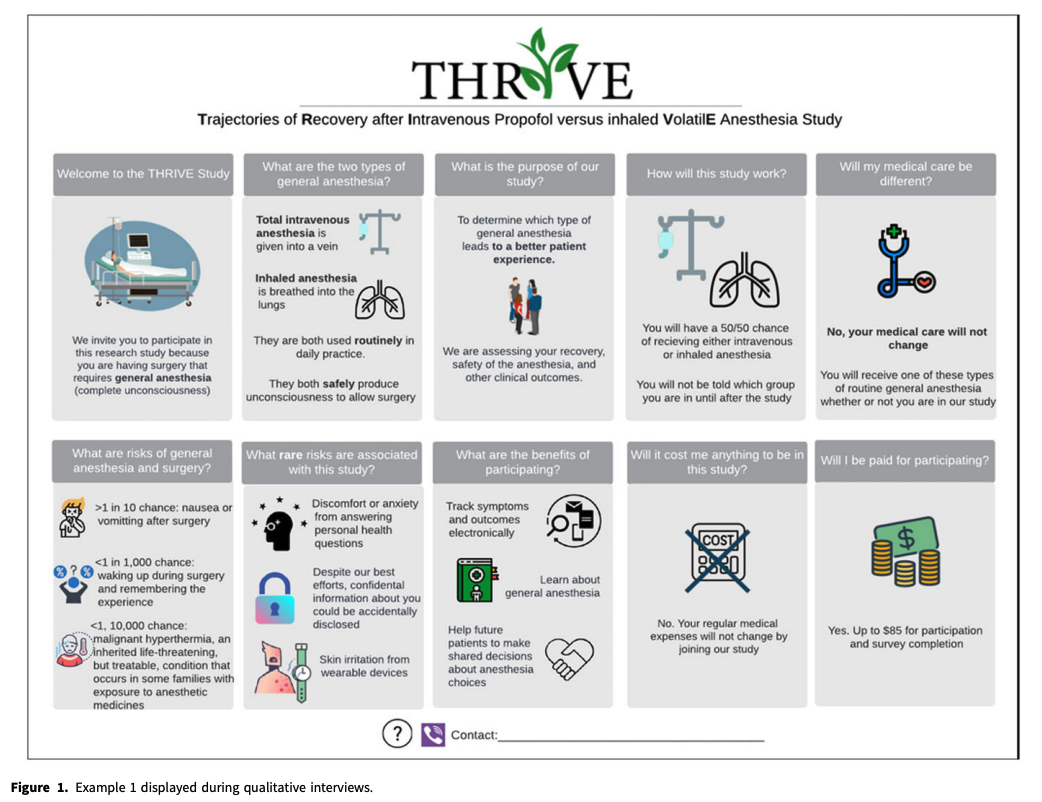
Editor’s note: This is an example of a visual KI sheet provided in the article.