Improving Comprehension of Consent Forms in Online Research: An Empirical Test of Four Interventions
Research Article
Naomi K. Grant, Leah K. Hamilton, Jenalyn M. Ormita
Journal of Empirical Research on Human Research Ethics, 14 March 2025
Open Access
Abstract
Informed consent is a guiding ethical principle when conducting research involving human participants. Yet, consent forms are often skimmed or ignored, jeopardizing informed consent. In two experiments, we test four interventions designed to encourage participants to read online consent forms more carefully. Experiment 1 employed a 2 (length: short or long) by 2 (timing: fixed or free) by 2 (quiz: present or absent) between-participants design. We measured instruction-following and comprehension of the consent form. Results showed that fixed timing and a quiz led to greater instruction-following, but consent form length had no effect. Experiment 2 employed a 2 (length: short or long) by 3 (delivery format: live, audiovisual, standard written) between-participants design. Once again, length had no effect, but both live and audiovisual formats increased instruction-following and comprehension. We recommend that researchers consider using fixed timing, adding a quiz, and/or using alternative delivery formats to help participants make an informed decision.
Category: Technology/Other Mediation
Pictorial Art for gaining Informed Consent in low-literacy settings
Pictorial Art for gaining Informed Consent in low-literacy settings
Swapnil.G. Ghotane, Clarice. Holt, Stephen.J. Challacombe, Patric. Don-Davis, David. Kamara, Jennifer.E. Gallagher
Patient Education and Counseling, 12 March 2025
Open Access
Abstract
Objective
Gaining informed consent for research in low-literacy setting is challenging. This study explores the creation and use of pictorial art in information and consent form in Sierra Leone (SL).
Methods
A pictorial ‘information and consent’ (PIC) sheet was developed with an illustrator (co-author) and local colleagues for a national oral health survey involving school children in SL. Evaluation included 500 participants (children and parents) who received a feedback form to assess their satisfaction with pictures and also their effectiveness in aiding understanding of the research process, using a visual five-point Likert scale. Data were descriptively analysed using STATA v.18.
Results
Feedback was received from 360 children (aged 12 and 15 years) and 14 parents of 6-year-olds. The average rating, out of five, for the question about liking pictures on the PIC sheet was 4.83 (S.D. = 0.62), while rating for how well pictures helped them understand the survey was 4.87 (S.D. = 0.54). Although most feedback was positive, a small minority expressed negative views.
Conclusion
Overall, participants appreciated that the pictorial aids had helped in understanding the research process.
Practical Implications
Pictorial aids show potential to improve comprehension and informed consent in low-literacy settings, indicating a promising approach for future research in similar contexts.
Supplementing Consent for a Prospective Longitudinal Cohort Study of Infants With Antenatal Opioid Exposure: Development and Assessment of a Digital Tool
Supplementing Consent for a Prospective Longitudinal Cohort Study of Infants With Antenatal Opioid Exposure: Development and Assessment of a Digital Tool
Jamie E Newman, Leslie Clarke, Pranav Athimuthu, Megan Dhawan, Sharon Owen, Traci Beiersdorfer, Lindsay M Parlberg, Ananta Bangdiwala, Taya McMillan, Sara B DeMauro, Scott Lorch, Myriam Peralta-Carcelen, Deanne Wilson-Costello, Namasivayam Ambalavanan, Stephanie L Merhar, Brenda Poindexter, Catherine Limperopoulos, Jonathan M Davis, Michele Walsh, Carla M Bann
JMIR Formative Research, 4 March 2025
Abstract
Background
The Outcomes of Babies With Opioid Exposure (OBOE) study is an observational cohort study examining the impact of antenatal opioid exposure on outcomes from birth to 2 years of age. COVID-19 social distancing measures presented challenges to research coordinators discussing the study at length with potential participants during the birth hospitalization, which impacted recruitment, particularly among caregivers of unexposed (control) infants. In response, the OBOE study developed a digital tool (consenter video) to supplement the informed consent process, make it more engaging, and foster greater identification with the research procedures among potential participants.
Objective
We aim to examine knowledge of the study, experiences with the consent process, and perceptions of the consenter video among potential participants of the OBOE study.
Methods
Analyses included 129 caregivers who were given the option to view the consenter video as a supplement to the consent process. Participants selected from 3 racially and ethnically diverse avatars to guide them through the 11-minute video with recorded voice-overs. After viewing the consenter video, participants completed a short survey to assess their knowledge of the study, experiences with the consent process, and perceptions of the tool, regardless of their decision to enroll in the main study. Chi-square tests were used to assess differences between caregivers of opioid-exposed and unexposed infants in survey responses and whether caregivers who selected avatars consistent with their racial or ethnic background were more likely to enroll in the study than those who selected avatars that were not consistent with their background.
Results
Participants demonstrated good understanding of the information presented, with 95% (n=123) correctly identifying the study purpose and 88% (n=112) correctly indicating that their infant would not be exposed to radiation during the magnetic resonance imaging. Nearly all indicated they were provided “just the right amount of information” (n=123, 98%) and that they understood the consent information well enough to decide whether to enroll (n=125, 97%). Survey responses were similar between caregivers of opioid-exposed infants and unexposed infants on all items except the decision to enroll. Those in the opioid-exposed group were more likely to enroll in the main study compared to the unexposed group (n=49, 89% vs n=38, 51%; P<.001). Of 81 caregivers with known race or ethnicity, 35 (43%) chose avatars to guide them through the video that matched their background. Caregivers selecting avatars consistent with their racial or ethnic background were more likely to enroll in the main study (n=29, 83% vs n=43, 57%; P=.01).
Conclusions
This interactive digital tool was helpful in informing prospective participants about the study. The consenter tool enhanced the informed consent process, reinforced why caregivers of unexposed infants were being approached, and was particularly helpful as a resource for families to understand magnetic resonance imaging procedures.
The Impact of Video Consent on Patient Satisfaction When Undergoing Percutaneous Nephrolithotomy: A Randomized Control Trial
The Impact of Video Consent on Patient Satisfaction When Undergoing Percutaneous Nephrolithotomy: A Randomized Control Trial
Kartik Sharma, Gautam Ram Choudhary, Shiv Charan Navriya, Jeena Raju Kudunthail, Deepak Prakash Bhirud, Mahendra Singh, Arjun Singh Sandhu
Société Internationale d’Urologie Journal, 12 February 2025
Abstract
Introduction
Consent-taking for surgery evolved from a historical paternalistic approach to informed consent in the mid-20th century. Modern healthcare models prioritize patient-centric care, and the use of multimedia tools may overcome challenges such as language barriers and complex medical surgical steps. This study evaluates the impact of an educational video on patient satisfaction for those undergoing percutaneous nephrolithotomy (PCNL), a procedure where explaining complexities verbally can be challenging.
Materials and Methods
A randomized control trial was conducted at a tertiary care center in India from July 2022 to April 2024. A total of 232 adult patients scheduled for PCNL were randomly assigned to a study group (Group A) or a control group (Group B). Group A viewed an educational video about PCNL, while Group B provided standard written consent. The video, presented in patients’ native languages, covered procedural details, potential outcomes, and post-operative care. Patient satisfaction was assessed using a 10-question questionnaire at discharge, with scores ranging from one (poor) to five (best). Statistical analysis was performed using SPSS software to compare outcomes between the two groups.
Results
The study found that Group A exhibited significantly higher satisfaction compared to Group B across all domains. Group A demonstrated a better understanding of the procedure, improved knowledge of post-operative care, reduced anxiety, and a greater awareness of potential complications. Specifically, the mean satisfaction scores for Group A were higher in understanding the procedure (13.15 vs. 10.00), post-operative care (8.46 vs. 6.84), and overall anxiety (8.65 vs. 6.96). The video also improved patients’ comprehension regarding potential complications and the need for further procedures. Complication rates and hospital stay durations were similar between both groups.
Discussion
The educational video significantly enhanced patient satisfaction and the understanding of PCNL. This multimedia approach provided a consistent, clear explanation of the procedure, which improved patient comprehension and reduced anxiety, irrespective of literacy levels. These findings support the integration of video-assisted consent in pre-operative education to enhance patient engagement and satisfaction.
Conclusions
The use of an educational video for consent in PCNL improves patient understanding and satisfaction. This method effectively complements traditional consent processes, providing a valuable tool for patient education in complex procedures.
Improving Endoscopy Room Efficiency: Evaluation of a Video as a Supplementary Tool for Informed Consent
Improving Endoscopy Room Efficiency: Evaluation of a Video as a Supplementary Tool for Informed Consent
A Kyei, O esenwa, C Tan, D Llovet, M Bernstein, B Mannino, L Cohen, N Griller, F Saibil, P Tartaro, E Yong, J Tinmouth
Journal of the Canadian Association of Gastroenterology, 10 February 2025
Abstract
Background
Endoscopy unit efficiency is critical because of the need to provide timely and quality care, despite limited resources. In previous work, obtaining informed consent negatively impacted efficiency. We developed a 3-minute animated video to facilitate the consent process, including describing colonoscopy, its purpose and potential risks/benefits.
Aims
1) Assess the ability of the video to support the informed consent process; 2) Determine the effectiveness of the video as a communication tool.
Methods
Using a critical case sample design with maximum variation, 12 participants completed pre- and post-colonoscopy 1:1 semi-structured interviews after viewing the video. Questions evaluated whether key components of informed consent were conveyed and assessed the video using principles of learner verification (attractiveness, usability, comprehension, impact on self-efficacy, acceptability). Interviews were recorded and transcribed. The data were coded inductively and deductively.
Results
Regarding components of informed consent, most participants understood the purpose and nature of a colonoscopy, but alternatives, including the right to refuse, were less effectively communicated. As a communication tool, the animations engaged participants and aided comprehension of complex material. The language was accessible, however, some participants found the video too fast and the font too small. Most participants found the video acceptable and characters relatable. Some identified information gaps included sedation level and procedure duration.
Conclusions
Endoscopy unit efficiency may be improved by providing consent information via video to patients scheduled for colonoscopy to supplement current approaches to informed consent. Our findings will inform revisions of the video and subsequent implementation into clinical practice.
How Inclusive Are Patient Decision Aids for People with Limited Health Literacy? An Analysis of Understandability Criteria and the Communication about Options and Probabilities
How Inclusive Are Patient Decision Aids for People with Limited Health Literacy? An Analysis of Understandability Criteria and the Communication about Options and Probabilities
Research Article
Romy Richter, Jesse Jansen, Josine van der Kraan, Wais Abbaspoor, Iris Bongaerts, Fleur Pouwels, Celine Vilters, Jany Rademakers, Trudy van der Weijden
Medical Decision Making, 14 December 2024
Abstract
Objective
Patient decision aids (PtDAs) can support shared decision making. We aimed to explore how inclusive PtDAs are for people with limited health literacy (LHL) by analyzing 1) the understandability of PtDAs using established criteria, 2) how options and probabilities of outcomes are communicated, and 3) the extent to which risk communication (RC) guidelines are followed.
Methods
In a descriptive document analysis, we analyzed Dutch PtDAs available in 2021 that met the International Patient Decision Aid Standards. We developed and pilot tested a data extraction form based on key RC and health literacy literature.
Results`
Most PtDAs (151/198) met most of the understandability criteria on layout (7–8 out of 8 items) such as font size but not on content aspects (121/198 PtDAs scored 5–7 out of 12 items) such as defining medical terms. Only 31 of 198 PtDAs used a short and simple sentence structure. Most PtDAs presented 2 to 4 treatment options. Many followed RC recommendations such as the use of numerical RC strategies such as percentages or natural frequencies (160/198) and visual formats such as icon arrays (91/198). Only 10 used neutral framing (10/198). When presented, uncertainty was presented verbally (134/198) or in ranges (58/198). Four PtDAs were co-created together with patients with LHL and used only verbal RC or no RC.
Conclusion
Most PtDAs met most of the understandability criteria on layout, but content aspects and adherence to RC strategies can be improved. Many PtDAs used long sentences and mostly verbal RC and are therefore likely to be inappropriate for patients with LHL. Further research is needed on PtDA characteristics and RC strategies suitable for people with LHL.
Highlights
- Despite meeting most criteria for understandability, many of the Dutch PtDAs use long sentences, which likely impede comprehension for patients with LHL.
- Most of the Dutch PtDAs follow established recommendations for risk communication, with room for improvement for some strategies such as framing and a clear reference to the time frame.
- Overall, more research is needed to tailor PtDAs to the needs of people with LHL.
Enhancing patient engagement: the influence of an in-consult patient decision aid on shared decision-making for lung tumour radiation – protocol for the randomised trial ‘SDM Lung SBRT’
Enhancing patient engagement: the influence of an in-consult patient decision aid on shared decision-making for lung tumour radiation – protocol for the randomised trial ‘SDM Lung SBRT’
Thomas Leth Fink, Torben Frøstrup Hansen, Charlotte Kristiansen, Torben Schjødt Hansen, Rune Slot Thing, Signe Timm, Karina Dahl Steffensen
BMJ Open: Randomized Controlled Trial, 20 January 2025; 15(1)
Abstract
Introduction
Patient engagement is continuously being promoted by patients as well as politicians and healthcare professionals. One way of increasing patient engagement is by using shared decision-making (SDM), which is a joint effort of clinicians and patients making decisions together. When planning stereotactic body radiation therapy (SBRT) for a lung tumour located close to the thoracic wall, there are conflicting interests between (1) delivering the highest possible dose to obtain local tumour control and (2) reducing the dose to the thoracic wall to decrease the risk of chest wall pain and rib fractures following treatment. The radiation oncologist often makes the choice of dose without any engagement of the patient. We believe that the patients should be engaged in such a decision. To explore this matter, we have designed a randomised trial, ‘SDM Lung SBRT’, for which we present our study protocol with a special focus on a patient decision aid (PtDA), which is being tested in this trial.
Methods and analysis
This study includes patients with a lung tumour located ≤1 cm from the thoracic wall. Patients are randomised to have the primary consultation with or without use of the PtDA. Treatment options are a radiation dose of either 66 Gray (Gy) in three fractions, 45 Gy in three fractions or no treatment. The primary outcome is patient engagement in decision-making measured by the validated observer-rated OPTION-12 score. Secondary outcomes are patient-reported outcomes, quality of life and side effects following treatment.
Ethics and dissemination
All patients give informed consent to participate. According to Danish legislation, ethical approval is not required for this study as studies using questionnaires, observations or other non-biological studies are not considered interventions according to the Committee Act. Results from this study will be presented at scientific meetings and published in English peer-reviewed journals.
Acceptability and effectiveness of a study information video in improving the research consent process for youth: a non-inferiority trial
Acceptability and effectiveness of a study information video in improving the research consent process for youth: a non-inferiority trial
Original Research
Tinashe Cynthia Mwaturura, Victoria Simms, Ethel Dauya, Som Kumar Shrestha, Salmaan Ferrand, Talent Shavani, Chido Dziva Chikwari, Constance R S Mackworth-Young, Tsitsi Bandason, Constancia Mavodza, Mandikudza Tembo, Katharina Kranzer, Sarah Bernays, Rashida Abbas Ferra
BMJ Global Health, 19 January 2025
Open Access
Abstract
Introduction
Obtaining informed consent for research includes the use of information sheets, which are often long and may be difficult for participants to understand. We conducted a trial to investigate whether consent procedures using a study information video coupled with electronic consent were non-inferior to standard consent procedures using participant information sheets (PIS) among youth aged 18–24 years in Zimbabwe.
Methods
The trial was nested within an endline population-based survey for a cluster-randomised trial from October 2021 to June 2022. Randomisation of participants to video or paper-based consent was at household level. We assessed non-inferiority in comprehension of the study using a questionnaire. The video method was accepted as non-inferior to standard consent procedures if the 95% CIs of the mean difference did not fall below the prespecified margin of 1.98. Thematic analysis was conducted on brief qualitative discussions with randomly selected youth to explore the acceptability of video and PIS within consent methods.
Results
Overall, 921 participants were enrolled (54% female). The median age was 20 (IQR 18–24) years. The mean comprehension score was 25.4/30 in both arms. The mean difference in comprehension between arms was −0.02 (95% CI −0.51 to 0.47) showing non-inferiority of the intervention in comprehension of study information. Youth (N=90) described both consent methods as interactive and inclusive. Those in the video consent arm felt it was exciting and youth focused. The use of imagery to explain procedures strengthened the perceived trustworthiness of the research. However, the high volume of information in both arms reduced acceptability.
Conclusion
Comprehension of study information using an information video is non-inferior to a paper-based consent method. Using information videos for consent processes shows promise as a person-centred and context-sensitive approach to enhance the informed consent process and should be encouraged by ethics committees.
Qualitative Assessment of Proposed Visual Key Information Pages for Informed Consent
Qualitative Assessment of Proposed Visual Key Information Pages for Informed Consent
Krista E. Cooksey, Eliana Goldstein, Clara Lee, Jessica Mozersky, Kimberly A. Kaphingst, Victor Catalan Gallegos, Mary C. Politi
Journal of Clinical and Translational Science, 21 November 2024
Open Access
Abstract
Introduction
The 2018 Common Rule revision intended to improve informed consent by recommending a concise key information (KI) section, yet provided little guidance about how to describe KI. We developed innovative, visual KI templates with attention to health literacy and visual design principles. We explored end users’ attitudes, beliefs, and institutional policies that could affect implementing visual KI pages.
Materials and Methods
From October 2023-April 2024, we conducted semi-structured interviews with principal investigators, research staff, institutional review board (IRB) personnel, including those in oversight/management, and community partners. 40 participants from 3 academic institutions (in the Midwest, Southeast, and Mountain West) viewed example KI pages and completed interviews. We coded written transcripts inductively and deductively based on the capability, opportunity, and motivation to change behavior (COM-B) framework. Data were analyzed using content analysis and organized thematically.
Results
Participants responded positively to the visual KI examples. They discussed potential benefits including improving information processing and understanding of study procedures, diversity in research, trust in research, and study workflow. They also described potential challenges to consider before widespread implementation: IRBs’ interpretations of federal guidelines, possible impact on the IRB submission processes, the effort/skill required to develop visuals, and difficulty succinctly communicating study risks. There was no consensus about when to use visual KI during consent, and some wondered if they were feasible for all study types.
Discussion
Visual KI offers a promising solution to long-standing informed consent challenges. Future work can explore resources and training to address challenges and promote widespread use.
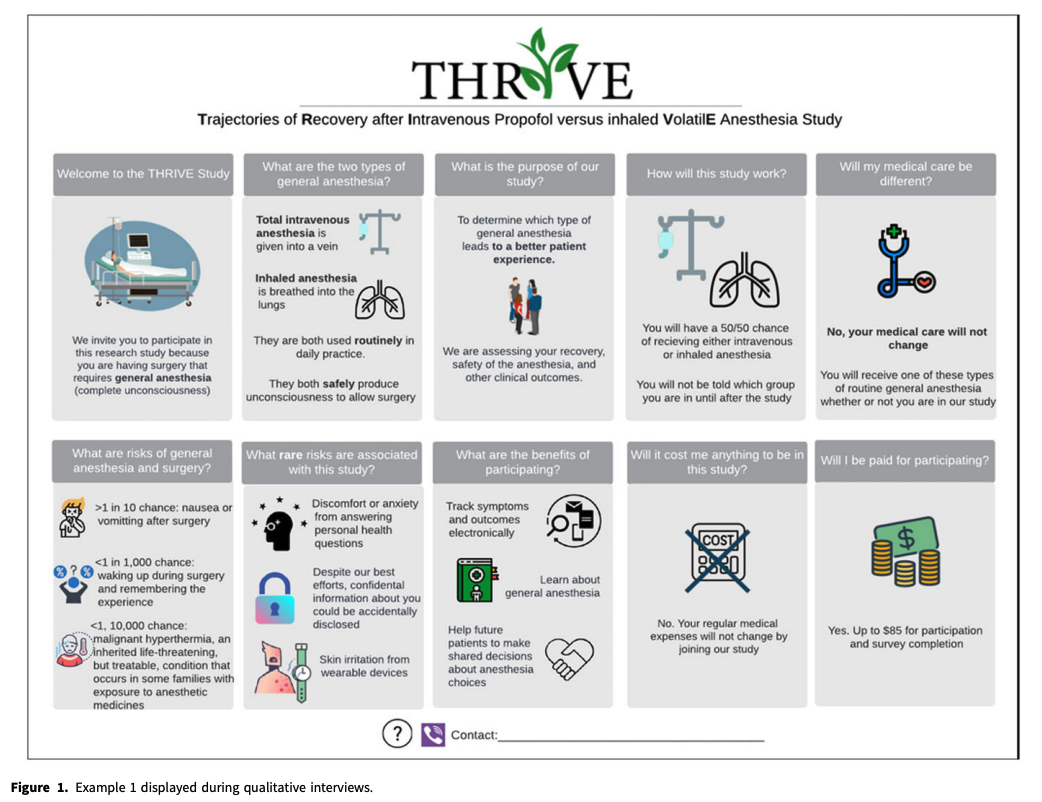
Editor’s note: This is an example of a visual KI sheet provided in the article.
Comparison Between Multimedia and Written Informed Consent for Lumbar Transforaminal Epidural Steroid Injection: A Randomized Controlled Pilot Trial
Comparison Between Multimedia and Written Informed Consent for Lumbar Transforaminal Epidural Steroid Injection: A Randomized Controlled Pilot Trial
Sunmin Kim, Nam Woo Kim, Francis Nahm, Eun Joo Choi, Pyung Bok Lee
Pain Physician, November 2024; 27(8) pp 529-535
Abstract
Background
Informed consent is a crucial ethical and legal requirement in medical practice to ensure that patients understand the risks, benefits, and alternatives of medical procedures. Recent advances in multimedia technology have facilitated the exploration of multimedia consent, aiming to enhance patient understanding and satisfaction. Ascertaining that patients have full comprehension of the procedures before opting to undergo them is especially important now that instances of such procedures as lumbar transforaminal epidural steroid injections (TESIs) are increasing.
Objectives
To determine the effectiveness of multimedia consent forms for lumbar transforaminal steroid injections.
Study design
Randomized clinical trial.
Setting
Outpatient multidisciplinary pain medicine center of a tertiary hospital.
Methods
A randomized controlled trial was conducted with 30 patients who received lumbar TESIs for lumbar radiculopathy. Patients were randomly assigned to either the multimedia consent group (Group M) or the conventional paper consent group (Group C). This study evaluated patients’ comprehension of the procedure, their anxiety levels (using the State-Trait Anxiety Inventory short form), and the patients’ post-procedure satisfaction.
Results
Group M showed significantly greater understanding of the procedure and reported lower levels of anxiety than did Group C (P = 0.041; P = 0.03). However, there were no statistically significant differences in post-procedure satisfaction between the groups (P = 0.25). These findings suggest that multimedia consent can effectively improve patient comprehension and reduce anxiety without significantly affecting patient satisfaction.
Limitations
First, the limited sample size of 30 patients restricts the applicability of our findings to a wider population, suggesting a need for larger studies to better assess the effects of multimedia consent. Second, conducting the study in a single hospital might have introduced bias. Multicenter research may provide a more diverse and accurate evaluation of the efficacy of multimedia consent.
Conclusion
This pilot study contributes to the growing evidence supporting the use of multimedia consent to enhance patient understanding and reduce anxiety, marking a promising direction for improving informed consent practices for less invasive procedures, such as lumbar TESIs. Further research is required to fully explore the benefits and limitations of multimedia consent forms in various medical settings.