Context is key: ethical considerations related to consent and study design in acute cardiac care research
Journal Article
Neal W Dickert, Madeline Meer
European Heart Journal Acute Cardiovascular Care, 28 November 2024
Excerpt
…There is also an important set of ethical issues that arise in cardiac critical care research. By their very nature, acute care studies involve ‘high stakes’ outcomes such as mortality, organ failure, and other major morbidities. Communicating about study enrolment with patients and family members in the context of life-or-death situations is difficult, and research itself is complex and unfamiliar. To make matters worse, decisions often must be made very quickly, because acute care must be delivered rapidly. These issues make consent processes difficult and, in some cases, impossible. Many patients with severe acute cardiac illness lack capacity to engage in decision-making, and surrogate decision makers are often unavailable and may struggle with having to make research enrolment decisions for someone else. The urgency of these situations only compounds baseline challenges related to deciphering patients’ preferences for participating in research.
In this piece, we focus on these ethical challenges and integrate them with practical considerations outlined above. We articulate paths forward for major types of acute cardiac care research, emphasising throughout the importance of attention to critical contextual factors…
On-site electronic consent in pediatrics using generic Informed Consent Service (gICS): Creating a specialized setup and collecting consent data
On-site electronic consent in pediatrics using generic Informed Consent Service (gICS): Creating a specialized setup and collecting consent data
Research Article
Katharina Danhauser, Larissa Dorothea Lina Mantoan, Jule Marie Dittmer, Simon Leutner, Stephan Endres, Karla Strniscak, Jenny Pfropfreis, Martin Bialke, Dana Stahl, Bernadette Anna Frey, Selina Sophie Gläser, Laura Aurica Ritter, Felix Linhardt, Bärbel Maag, Georgia Donata Emily Miebach, Mirjam Schäfer, Christoph Klein, Ludwig Christian Hinske
PLOS Digital Health, 25 November 2024
Open Access
Abstract
Enrolling in a clinical trial or study requires informed consent. Furthermore, it is crucial to ensure proper consent when storing samples in biobanks for future research, as these samples may be used in studies beyond their initial purpose. For pediatric studies, consent must be obtained from both the child and their legal guardians, requiring the recording of multiple consents at once. Electronic consent has become more popular recently due to its ability to prevent errors and simplify the documentation of multiple consents. However, integrating consent capture into existing study software structures remains a challenge. This report evaluates the usability of the generic Informed Consent Service (gICS) of the University Medicine Greifswald (UMG) for obtaining electronic consent in pediatric studies. The setup was designed to integrate seamlessly with the current infrastructure and meet the specific needs of a multi-user, multi-study environment. The study was conducted in a pediatric research setting, where additional informed consent was obtained separately for the biobank. Over a period of 54 weeks, 1061 children and adolescents aged 3 to 17 years participated in the study. Out of these, 348 agreed also to participate in the biobank. The analysis included a total of 2066 consents and assents, with 945 paper-based and 1121 electronic consents. The study assessed the error susceptibility of electronic versus paper-based consents and found a significant reduction rate of errors of 94.7%. These findings provide valuable insights into the use of gICS in various studies and the practical implementation of electronic consent software in pediatric medicine.
“You knew what you were getting into”: Perspective differences in gauging informed consent
“You knew what you were getting into”: Perspective differences in gauging informed consent
Rachel Schlund, Vanessa K. Bohns
Organizational Behavior and Human Decision Processes, January 2025
Abstract
We examine differences between perceived and experienced consent in organizational contexts—specifically, the aspect of consent that reflects how informed consenters feel. We theorize that people tasked with soliciting consent overestimate the extent to which consenters feel fully informed of what they are agreeing to and thus feel they have truly consented. We provide support for these predictions across six pre-registered studies (N = 2,993) and eight supplemental pre-registered studies (N = 4,406) that establish causal and mediation evidence, downstream organizational consequences, and real-world relevance. This research reveals that even when an agreement meets the legal criteria for consent, there may be misaligned perceptions of employees’ feelings of consent, with consequences for employees’ relationship with their organization. The current studies offer a significant step forward in understanding the markedly understudied role of consent in organizations.
Ethical Challenges in the Integration of Artificial Intelligence in Palliative Care
Ethical Challenges in the Integration of Artificial Intelligence in Palliative Care
Abiodun Adegbesan, Adewunmi Akingbola, Olajide Ojo, Otumara Urowoli Jessica, Uthman Hassan Alao, Uchechukwu Shagaya, Olajumoke Adewole, Owolabi Abdullahi
Journal of Medicine, Surgery, and Public Health, December 2024
Abstract
The integration of artificial intelligence (AI) into palliative care offers the possibility of improved patient outcomes through enhanced decision-making, personalized care, and reduced healthcare provider burden. However, the use of AI in this sensitive area presents significant ethical challenges which require serious consideration to ensure that technology serves the best interests of patients without compromising their rights or well-being. This narrative review explores the key ethical issues associated with AI in palliative care, with a focus on low-resource settings where these challenges are often intensified. The review examines essential ethical principles such as autonomy, beneficence, non-maleficence, and justice, and identifies critical concerns including data privacy, informed consent, algorithmic bias, and the risk of depersonalizing care. It also highlights the unique difficulties faced in low-resource environments, where the lack of infrastructure and regulatory frameworks can exacerbate these ethical risks. To address these challenges, the review offers actionable recommendations, such as developing context-specific guidelines, promoting transparency and accountability through explainable AI (XAI), and conducting regular ethical audits. Interdisciplinary collaboration is emphasized to ensure that AI systems are ethically designed and implemented, respecting cultural contexts and upholding patient dignity. This study contributes to the ongoing discourse on ethical AI integration in healthcare, indicating the need for careful consideration of ethical principles to ensure that AI enhances rather than undermines the compassionate care at the heart of palliative care. These findings serve as a foundation for future research and policy development in this emerging field.
Ethical Considerations in Using AI for Mental Health Diagnosis and Treatment Planning: A Scoping Review
Ethical Considerations in Using AI for Mental Health Diagnosis and Treatment Planning: A Scoping Review
Yewande Ojo
Proceedings of the International Conference on Artificial Intelligence and Robotics; Yaba Nigeria, 26-28 November 2024
Abstract
Integrating Artificial Intelligence (AI) with mental healthcare presents a paradigm shift in diagnosis and treatment planning, offering potential efficiency, accuracy, and personalisation improvements. However, this technological advancement allows for the exploration of a complex array of ethical challenges that demand careful consideration. This research explores the vital ethical dimensions surrounding the adoption of AI in mental health contexts, emphasising the reason for a balanced approach that maximises benefits while mitigating risks.
Central to these considerations is the imperative of privacy and data protection. This type of mental health information requires comprehensive robust safeguards to prevent unauthorised access or misuse while allowing for responsible data utilisation to drive AI-powered advancements. The assurance of fairness and non-discrimination in AI systems is critical, as racial bias could exacerbate disparities in mental healthcare access and outcomes. Transparency and explainability emerge as crucial factors in fostering trust and accountability. AI systems must be capable of providing clear rationales for their diagnostic and proposed treatment planning, which aids clinicians and patients to make informed decisions. This transparency is intimately linked to the principles of autonomy and informed consent, requiring that individuals fully understand the role of AI in their treatment and have the agency to accept or decline its use.
The integration of AI also necessitates a reevaluation of professional ethics and responsibilities for mental health practitioners. As AI systems assume more significant roles in diagnosis and treatment planning, the boundaries of professional judgment and accountability must be delineated. Moreover, the broader societal implications, including potential changes in public perception of mental healthcare and shifts in the healthcare workforce, warrant careful consideration.
Regulatory and governance frameworks play a pivotal role in addressing these ethical challenges. Policymakers face the complex task of developing adaptive regulations that foster innovation while ensuring robust ethical safeguards. This requires a collaborative approach involving clinicians, researchers, ethicists, patients, and technology developers.
Qualitative Assessment of Proposed Visual Key Information Pages for Informed Consent
Qualitative Assessment of Proposed Visual Key Information Pages for Informed Consent
Krista E. Cooksey, Eliana Goldstein, Clara Lee, Jessica Mozersky, Kimberly A. Kaphingst, Victor Catalan Gallegos, Mary C. Politi
Journal of Clinical and Translational Science, 21 November 2024
Open Access
Abstract
Introduction
The 2018 Common Rule revision intended to improve informed consent by recommending a concise key information (KI) section, yet provided little guidance about how to describe KI. We developed innovative, visual KI templates with attention to health literacy and visual design principles. We explored end users’ attitudes, beliefs, and institutional policies that could affect implementing visual KI pages.
Materials and Methods
From October 2023-April 2024, we conducted semi-structured interviews with principal investigators, research staff, institutional review board (IRB) personnel, including those in oversight/management, and community partners. 40 participants from 3 academic institutions (in the Midwest, Southeast, and Mountain West) viewed example KI pages and completed interviews. We coded written transcripts inductively and deductively based on the capability, opportunity, and motivation to change behavior (COM-B) framework. Data were analyzed using content analysis and organized thematically.
Results
Participants responded positively to the visual KI examples. They discussed potential benefits including improving information processing and understanding of study procedures, diversity in research, trust in research, and study workflow. They also described potential challenges to consider before widespread implementation: IRBs’ interpretations of federal guidelines, possible impact on the IRB submission processes, the effort/skill required to develop visuals, and difficulty succinctly communicating study risks. There was no consensus about when to use visual KI during consent, and some wondered if they were feasible for all study types.
Discussion
Visual KI offers a promising solution to long-standing informed consent challenges. Future work can explore resources and training to address challenges and promote widespread use.
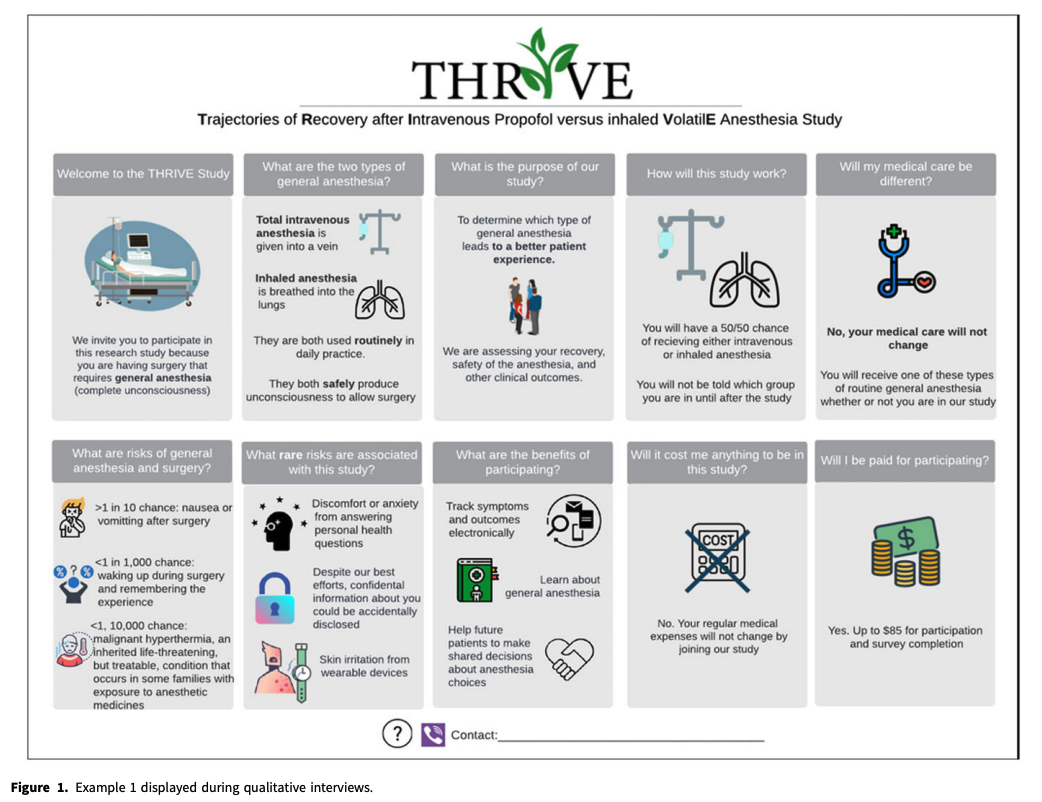
Editor’s note: This is an example of a visual KI sheet provided in the article.
Comparison Between Multimedia and Written Informed Consent for Lumbar Transforaminal Epidural Steroid Injection: A Randomized Controlled Pilot Trial
Comparison Between Multimedia and Written Informed Consent for Lumbar Transforaminal Epidural Steroid Injection: A Randomized Controlled Pilot Trial
Sunmin Kim, Nam Woo Kim, Francis Nahm, Eun Joo Choi, Pyung Bok Lee
Pain Physician, November 2024; 27(8) pp 529-535
Abstract
Background
Informed consent is a crucial ethical and legal requirement in medical practice to ensure that patients understand the risks, benefits, and alternatives of medical procedures. Recent advances in multimedia technology have facilitated the exploration of multimedia consent, aiming to enhance patient understanding and satisfaction. Ascertaining that patients have full comprehension of the procedures before opting to undergo them is especially important now that instances of such procedures as lumbar transforaminal epidural steroid injections (TESIs) are increasing.
Objectives
To determine the effectiveness of multimedia consent forms for lumbar transforaminal steroid injections.
Study design
Randomized clinical trial.
Setting
Outpatient multidisciplinary pain medicine center of a tertiary hospital.
Methods
A randomized controlled trial was conducted with 30 patients who received lumbar TESIs for lumbar radiculopathy. Patients were randomly assigned to either the multimedia consent group (Group M) or the conventional paper consent group (Group C). This study evaluated patients’ comprehension of the procedure, their anxiety levels (using the State-Trait Anxiety Inventory short form), and the patients’ post-procedure satisfaction.
Results
Group M showed significantly greater understanding of the procedure and reported lower levels of anxiety than did Group C (P = 0.041; P = 0.03). However, there were no statistically significant differences in post-procedure satisfaction between the groups (P = 0.25). These findings suggest that multimedia consent can effectively improve patient comprehension and reduce anxiety without significantly affecting patient satisfaction.
Limitations
First, the limited sample size of 30 patients restricts the applicability of our findings to a wider population, suggesting a need for larger studies to better assess the effects of multimedia consent. Second, conducting the study in a single hospital might have introduced bias. Multicenter research may provide a more diverse and accurate evaluation of the efficacy of multimedia consent.
Conclusion
This pilot study contributes to the growing evidence supporting the use of multimedia consent to enhance patient understanding and reduce anxiety, marking a promising direction for improving informed consent practices for less invasive procedures, such as lumbar TESIs. Further research is required to fully explore the benefits and limitations of multimedia consent forms in various medical settings.
The Impact of Artistic Representation on Patient Consent and Autonomy
The Impact of Artistic Representation on Patient Consent and Autonomy
Alberta Jeanne N.
Eurasian Experiment Journal of Scientific and Applied Research, 2024; 6(2)
Open Access
Abstract
Informed consent is fundamental to respecting patient autonomy in healthcare, ensuring that patients make voluntary, well-informed decisions about their treatment. However, consent processes can be hindered by communication barriers, time constraints, and differences in understanding complex medical information. Artistic representations in healthcare ranging from visual arts and music to theater—have emerged as potential tools to enhance patient comprehension and emotional engagement with medical procedures. This paper examines how various forms of art in healthcare environments may influence patient consent and autonomy. It considers the psychological, communicative, and therapeutic impacts of art, proposing that art can support consent by fostering deeper understanding and empathy between patients and healthcare providers. Four theoretical frameworks psychological impact, communicative theory, therapeutic influence, and autonomy in healthcare are analyzed to assess the efficacy of artistic representations in supporting patient-centered consent. By synthesizing empirical studies on artistic strategies in clinical settings, this article highlights the importance of interdisciplinary approaches to patient autonomy and explores the ethical implications of integrating art into consent processes.
Smart contract empowered dynamic consent: decentralized storage and access control for healthcare applications
Smart contract empowered dynamic consent: decentralized storage and access control for healthcare applications
Aparna Singh, Geetanjali Rathee
Peer-to-Peer Networking and Applications, 10 December 2024
Abstract
The ability to share Electronic Health Records (EHRs) with the right people plays a crucial role in providing on-time diagnosis. The outsourcing of private health data on the cloud, with negligible control in the hands of the data owners, poses a significant challenge for e-healthcare applications. Obtaining the owner’s consent is not just essential for data sharing but also to protect the privacy of the data being shared. With the help of Blockchain and Interplanetary File System (IPFS), this research paper proposes a purpose-based framework for obtaining dynamic consent before sharing data among healthcare providers, academicians, etc. The study develops a blockchain system on the Ethereum network to store encrypted EHRs on IPFS. This architecture allows for secure and privacy-enhanced data sharing. The proposed model demonstrates effectiveness in enhancing data security, privacy, scalability, ownership, and integrity of medical records. The cost analysis shows a negligible contract deployment cost of 0.00174363 ETH, equivalent to $2.78. The framework presents a viable solution for securing EHRs and obtaining dynamic consent, ensuring improved healthcare data management. The use of blockchain and IPFS offers a promising avenue for enhancing data security and privacy in e-healthcare applications.
Stakeholder Perspectives on Research Consent and Reconsent for Procedures Involving Biological Samples and Biobanking of Children and Adolescents Living With HIV in Kenya
Stakeholder Perspectives on Research Consent and Reconsent for Procedures Involving Biological Samples and Biobanking of Children and Adolescents Living With HIV in Kenya
Research article
Josephine Aluoch, Ashley Chory, Michael Scanlon, Emma Gillette, Hillary Koros, Dennis Munyoro, Celestine Ashimosi, Whitney Beigon, Janet Lidweye, Jack Nyagaya, Allison DeLong, Rami Kantor, Rachel Christine Vreeman, Violet Naanyu, Winstone M. Nyandiko
Journal of the International Association of Providers of AIDS Care, 13 December 2024
Open Access
Abstract
Objective
To explore the perspectives of stakeholders on consenting and reconsenting children and adolescents living with HIV (CALWH) to participate in research involving biological sampling and biobanking. Stakeholders included CALWH, their caregivers, subject matter experts (SMEs) such as Institutional Review Board (IRB) members, Community Advisory Board (CAB) members, Healthcare Providers, researchers, and community leaders.
Study design
This qualitative study was conducted at the Academic Model Providing Access to Healthcare (AMPATH) in Kenya. Semi-structured interviews were conducted with CALWH, their caregivers, and SMEs. Audio recordings were transcribed, thematically analyzed, and emerging themes derived.
Results
In total, 99 participants were interviewed, of which the majority (52%) were female; 50% of CALWH were female with a median age of 17.5 years (range 11-24); 70% of caregivers and 44% of SMEs were female. All SMEs, CALWH, and caregivers emphasized that recontacting and reconsenting were their strong preferences for the use of biospecimens and also an essential procedure to address legal and ethical considerations and confidentiality. All CALWH wanted consent to detail how they will be informed about research findings and emphasized making their results available to them. Caregivers highlighted the importance of trust in the use of the stored samples to be maintained as per the consents.
Conclusion
Our findings revealed that CALWH and their caregivers want researchers to go beyond the typical information provided about biospecimen storage and use. They desire to be recontacted and reconsented as well as maintain ongoing communication with the research team about the research findings.